
SUPERA KVALITETA provides business consulting services in the field of medical devices
- implementation of quality management system according to the requirements of ISO 13485:2016 in:
- production
- warehousing
- servicing of medical devices
- implementation, maintenance and improvment of
- Good Manufacturing Practice - GMP
- Good Distribution Practice - GDP
- preparation of product technical documentation including Declaration of Conformity
- clinical evaluation
We also perform internal audits and third-party audits in compliance with applicable regulatory requirements and standards.
We organize and perform in-house trainings in English language for GxP, CE marking and other topics in the field of medical devices.

SUPERA KVALITETA provides business consulting services related to statistical data processing and statistical data processing itself for various needs:
- resolving deviations and corrective actions
- process and product validation
- process development, improvement and optimization
- preparing annual quality review
- processing the results of stability studies
- process and final control data processing
- statistical processing for the purposes of scientific and other projects
- statistical planning of experiments (Design of Experiment) and statistical processing of the results obtained
- determining the required sample size and preparing sampling plans according to an acceptable quality level
For statistical data processing we use the licensed software STATISTICA.
STATISTICA software is validated and recognized by the US Food and Drug Administration (FDA) and other regulatory institutions.
Statistical data processing includes:
- transfer of data from Excel to STATISTICA - if the data are not yet entered to Excel, we send the table format for entering the necessary data
- transfer of statistical results (tables and graphs) from STATISTICA to Word
- interpretaciju dobivenih statističkih rezultata, komentare, i objašnjenja
- interpretation of obtained statistical results, comments, and explanations
- additional consultation, if needed
We have done numerous statistical processing for doctoral dissertations, scientific and professional papers in the field of medicine, pharmaceutics, dentistry, veterinary, biochemistry, food technology, economics, marketing, agronomy, psychology.
We organize and perform in-house trainings in English language in the field of statistics.
SUPERA KVALITETA provides business consulting services for activities related to:
computer system validation, small application validation, cleaning validation, process validation, equipment qualification
 Validation of information, laboratory and process systems and maintenance of computer systems in a validated state in compliance with applicable guidelines and guides: EU GMP Annex 11, ISPE GAMP 5, FDA 21 CFR Part 11 (for systems using electronic records and signatures) covers activities:
Validation of information, laboratory and process systems and maintenance of computer systems in a validated state in compliance with applicable guidelines and guides: EU GMP Annex 11, ISPE GAMP 5, FDA 21 CFR Part 11 (for systems using electronic records and signatures) covers activities:
- assistance in the preparation and / or review of validation documentation for all phases of the computer system life cycle (planning, specifications, development, installation qualification, operation qualification, performance qualification, "go-live")
- assistance in producing the necessary procedures (SOP - Standard Operating Procedure)
- risk analysis
- audit of vendor
- performing education
 We provide support for the creation and validation of small applications (Access, Excel, FileMaker) used in the GxP environment that must be validated.
We provide support for the creation and validation of small applications (Access, Excel, FileMaker) used in the GxP environment that must be validated.
 We also provide support services for compliance of systems, computer systems and procedures with data integrity requirements in accordance with applicable guidelines.
We also provide support services for compliance of systems, computer systems and procedures with data integrity requirements in accordance with applicable guidelines.
 Cleaning validation - the purpose of cleaning validation is to confirm that the cleaning process is effective and to ensure that the medicinal product is safe by preventing cross-contamination.
Cleaning validation - the purpose of cleaning validation is to confirm that the cleaning process is effective and to ensure that the medicinal product is safe by preventing cross-contamination.
Ceaning validation and maintenance of validated status in compliance with applicable Guidelines and Guides: EU GMP Vol 4. Part I and Part II, EU GMP Annex 15, PIC / S PI 006, APIC Guidance on aspects of cleaning validation in active pharmaceutical ingredient plants, FDA Guide to inspections validation of cleaning processes, ICH Guidelines, VICH Guidelines, covers activities:
- assistance in preparation and / or review of validation documentation (validation master plan (VMP), cleaning validation protocol, cleaning validation report, descriptions of cleaning procedures - SOPs, work instructions, etc.)
- pomoć u izradi matrice opreme proizvoda i grupiranju proizvoda
- assistance in preparation of product equipment matrix and product grouping
- assistance to set eligibility criteria and to calculate limits for the rest of the product
- risk analysis
- performing education
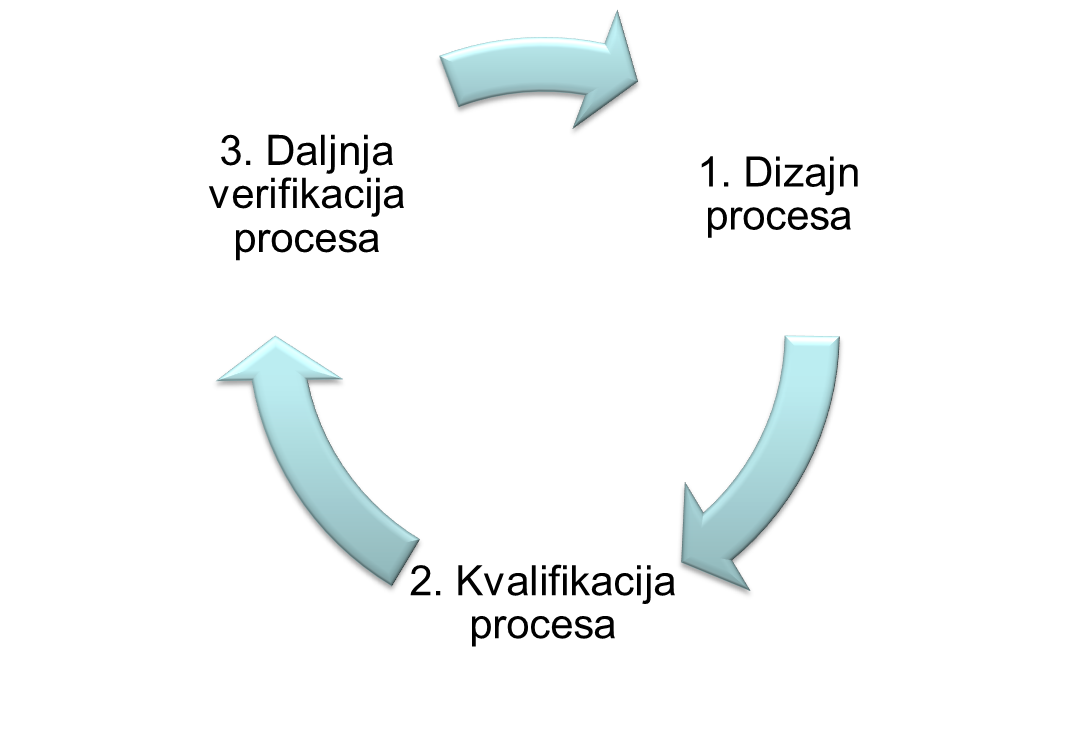
Process validation - documented evidence that a process run within set parameters can effectively and reproducibly produce a product that complies with predefined specifications and quality characteristics.
Process validation in accordance with applicable guidelines and guides: EU GMP Annex 15, EU GMP Chapter 5, EMA Guideline on Process Validation, FDA Guidance for Industry Process Validation, includes activities:
- assistance in the preparation and / or review of validation documentation (validation master plan (VMP), validation protocol (validation plan), validation report)
- risk analysis
- consulting in the field of modern approaches to process development and validation: Quality by Design (QbD), Process Analytical Technology (PAT), Continued Process Verification
- performing education
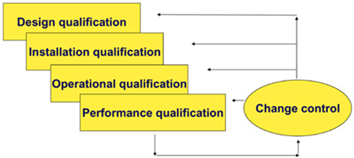 Equipment qualification - process that proves and documents that the equipment is functioning properly and that it really produces the expected results.
Equipment qualification - process that proves and documents that the equipment is functioning properly and that it really produces the expected results.
Equipment qualification in accordance with applicable guidelines and guides: EU GMP Vol 4. Part I and Part II, EU GMP Annex 15, EMA Guideline on Process Validation, PIC / S PI 006, WHO Annex 4 - Appendix 6, FDA Guidance for Industry Process Validation, includes activities:
- assistance in the preparation and / or review of qualification documentation (User Requirements Specification (URS), Design Qualification - DQ), Factory Acceptance Testing (FAT), Site Acceptance Testing - SAT), Installation Qualification - IQ, Operational Qualification - OQ, Performance Qualification - PQ
- risk analysis
- performing education
We organize and perform in-house trainings in English language for computer system validation, small application validation, cleaning validation, process validation, equipment qualification and other similar topics.




SUPERA KVALITETA offers representation service to companies located outside the EU in the process of submitting the application for the Good Manufacturing Practice Certificate to the Agency for Medicinal Products and Medical Devices of Croatia (HALMED) in Zagreb, Croatia as well as its services in communication with the HALMED in the process of obtaining the Good Manufacturing Practice Certificate.
In Europe, manufacturers of medicinal products and active pharmaceutical ingredients (APIs) are subject to monitoring by competent authorities with regard to compliance with the principles of Good Manufacturing Practices (GMP). Each EU Member State disposes of its own inspectorates. The location of the production is therefore crucial in order to decide which inspectorate is responsible.
The competent authority of each Member State carries out its inspections based on the requirements laid down in EU Directive 2003/94. The concrete interpretation of these requirements can be found in the EU GMP Guide and its Annexes.
If the manufacturing site is located outside the EU, it is important to verify into which country the medicinal product will be imported (the first entry to the EU market defines the competent authority).
To import a medicinal product or APIs, a company located outside the EU needs an import authorization (which includes a permanent representative in Europe). Prior to the first importation of medicinal products or APIs (and in order to receive the import licence), the site located outside the EU has to be inspected by a competent EU authority. A GMP inspection by an EU authority will be performed when the manufacturing site outside the EU either has a valid marketing authorization for a medicinal product or APIs (issued by a competent EU authority or by EMA) or if it is referenced by an EU Marketing Authorization Holder for manufacturing.
In order to obtain a GMP certificate an Importer or a Marketing Authorization Holder should apply for a GMP inspection of a manufacturing site in a third country.
It may be possible to request an inspection on a voluntary basis. To explore this possibility, the authorities of the Member State into which the product will be imported into the EEA should be contacted.
If you do not have a permanent representative or Marketing Authorization Holder in Europe, you will need a representative in the EU to submit this application.




An independent audit (assessment, inspection) is a tool that will help you to evaluate objectively the current status of your company, laboratory, or institution in compliance with applicable regulatory requirements and standards.
SUPERA KVALITETA provides internal auditing services for your company, laboratory or institution, as well as audits of your suppliers, contracted manufacturers, laboratories or partners.
We perform audits in an open and positive manner, in a time and volume appropriate to your needs, with assured objectivity, experience and confidentiality. Upon completion of the audit, we submit the Audit Report.
We offer the possibility of monitoring the results of the audits performed and for resolving non-conformities (discrepancies) observed. We produce regular periodic reports that include the status of implementation and the checking of corrective and preventive actions efficiency to ensure that all audits are properly completed.
To API manufacturers and wholesalers we offer an independent audit. We sell the audit report to partners / customers / suppliers of those manufacturers or wholesalers. This way manufacturers and wholesalers reduce their costs, optimize their time and reduce the pressure / stress that comes with the auditors from different companies, who conduct the same type of audits. On the other hand, their partners / customers / suppliers also reduce their costs and optimize their time, because they can use the time spent on auditing and writing reports for other business activities.
SUPERA KVALITETA performs:
- Third-party audits
We perform audits of your suppliers, contracted manufacturers, or laboratories in compliance with applicable regulatory requirements and standards.
- Internal audits
We perform internal audits in your company, laboratory or institution in compliance with applicable regulatory requirements and standards.
- "Mock Regulatory" audits
Clients receive practical simulation of regulatory inspection and advices on how to manage during regulatory inspection and how to present inspected areas.
- "Benchmarking" audits
We help you to evaluate the system in place in your company in regard to others in the market and to give you practical recommendations on how to improve the system.
- "Due Diligence" audits
We perform a complete and detailed assessment of compliance with the regulatory requirements and standards of the company or project of your interest.
According to your needs and interest we organize and perform in-house trainings for internal auditors.
